Built for Pharma.
Powered by AIM | Pace™
Quick to integrate production planning that fits your plant, not the other way around.
Most planning tools aren't built for pharma. We are.
Every dosage form comes with its own planning complexity, and we support them all. AIM | Pace adapts to the GMP rules, equipment constraints, and team structures that define how you manufacture. From injectable lines to oral solids and veterinary suites, our engine keeps your schedule compliant, stable, and executable.
 See how
See howAIM | Pace™
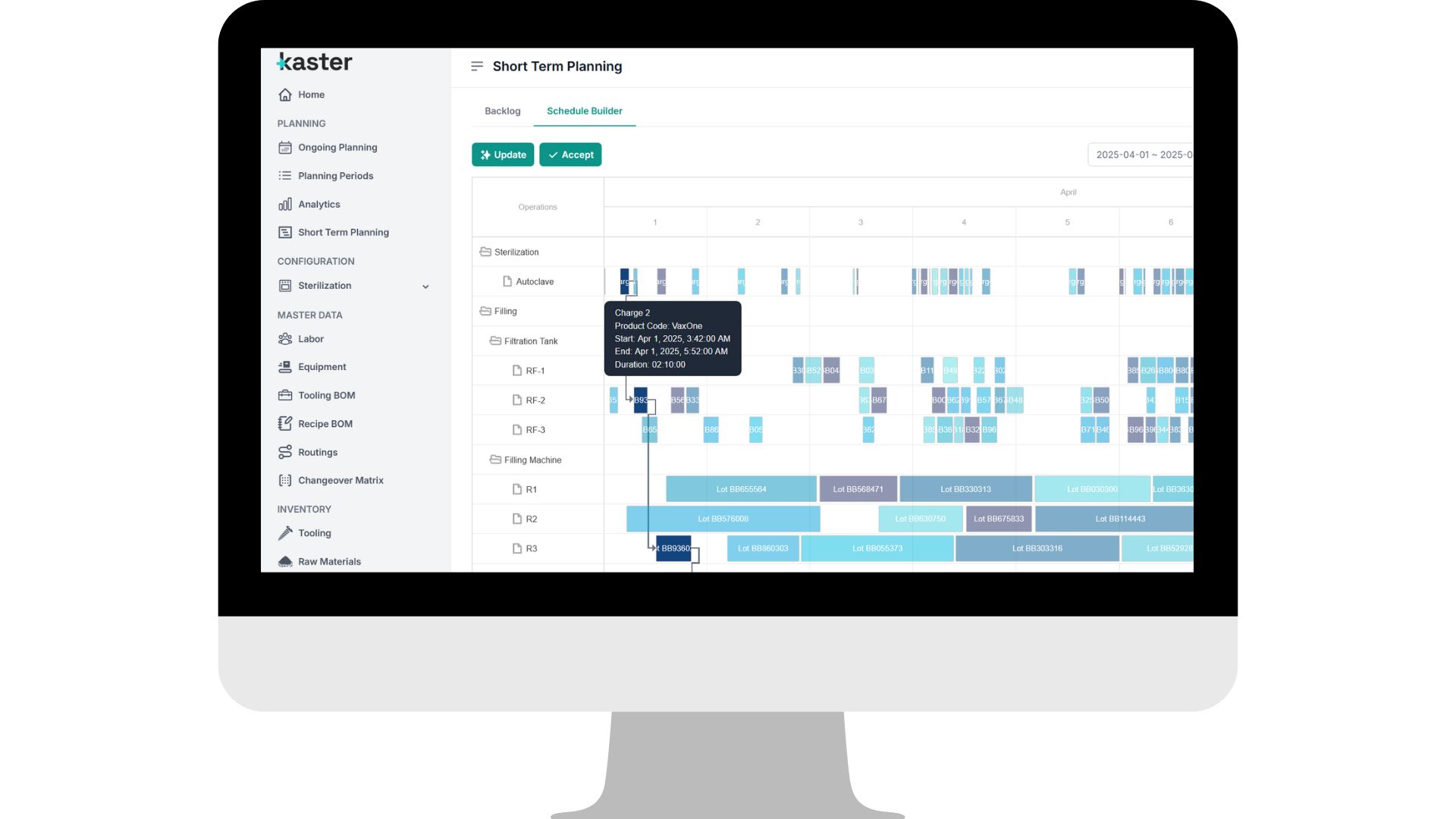
Coordinating changeovers, release timings, shared resources, and shifting priorities challenges even the best-run plants.
AIM | Pace turns that complexity into GMP-compliant daily and weekly production plans that adapt in real time to shop-floor realities.
Built for sterile injectables, OSD, biologics, non-sterile liquids, suspensions, creams, and more, it delivers stable operations, higher throughput, and reduced overtime, all without added CapEx.
 See it in action
See it in actionExplore by production type
Each drug format comes with its own scheduling hurdles and coordination demands. With so many constraints; equipment, cleaning, testing, labor, conformities, deadlines and priorities, planners are expected to remember and manage everything at once. We built the systems that helps simplify that reality, adapting to each production type to keep schedules clear, compliant, and under control.

Built for Compliance,
Across All Forms
No matter the dosage form, every schedule is GMP-aware by design and delivers fast ROI:

+10% Capacity Gain, unlocked without CapEx.

+20% more efficient workflows, validated at Canada’s largest sterile injectable site.

GMP-Compliant, Audit-Ready Execution, confidence in every plan.
One Platform, Any Environment.
AIM Pace adapts to your operations:

Multi-product, multi-line CDMOs

Hybrid high-throughput sites producing human & veterinary drugs

Batch-based or campaign-based scheduling

Sustainability & ESG contribution

Equipment sitting idle, unnecessary cleaning, or night shift overruns don’t just cost time, they consume energy, ingredients, and human resources. AIM | Pace helps reduce this waste by improving plan accuracy, minimizing avoidable cleaning, and reducing equipment overuse.

Why partner with us
We don’t just provide solutions to your toughest problems, we build long lasting innovation partnerships.

Co-develop with your teams.
We work side-by-side with your planners, operators, and quality teams to shape features that address real-world bottlenecks and fit seamlessly into your workflows.

Applied Research Pipeline
We continuously turns frontline manufacturing challenges, from changeovers to lab release sync, into deployable features that evolve with your operations.

Built-In GMP & Pharmaceutical Expertise
Purpose-built for sterile injectables, OSD, biologics, and more. Every feature is designed for large-scale workflows with compliance at the core.
Ready to see how AIM | Pace fits your plant?
Let’s walk through how we schedule exactly what you produce, with the constraints you live with every day.

