Solving the operational problems no one else wants to touch.
We don’t replace your systems, we fix what falls between them.
Pharmaceutical manufacturing is one of the most complex, compliance-driven industries in the world.
Behind every delay, deviation, or rework hour is the same root issue: planning doesn’t reflect how your plant actually runs. We solve the problems that spreadsheets, ERPs, MES and other platforms can't, the ones that cost you time, capacity, and trust.
 See how
See howWhy planning breaks in pharma
No matter the dosage form, every schedule is GMP-aware by design and delivers fast ROI:

Plans aren’t stable. They don’t hold beyond 24–48 hours.

People, equipment, and QA aren't aligned. Everyone works hard, but not together.

Constraints aren’t visible. Cleaning, QA, process timings, and material status are handled offline.

Teams plan in silos. Operations, planning, QA, and packaging use different tools and calendars.

When it breaks, it breaks big. A single shift change, bad operation sync, or QA hold derails the week and can lead to shortages.
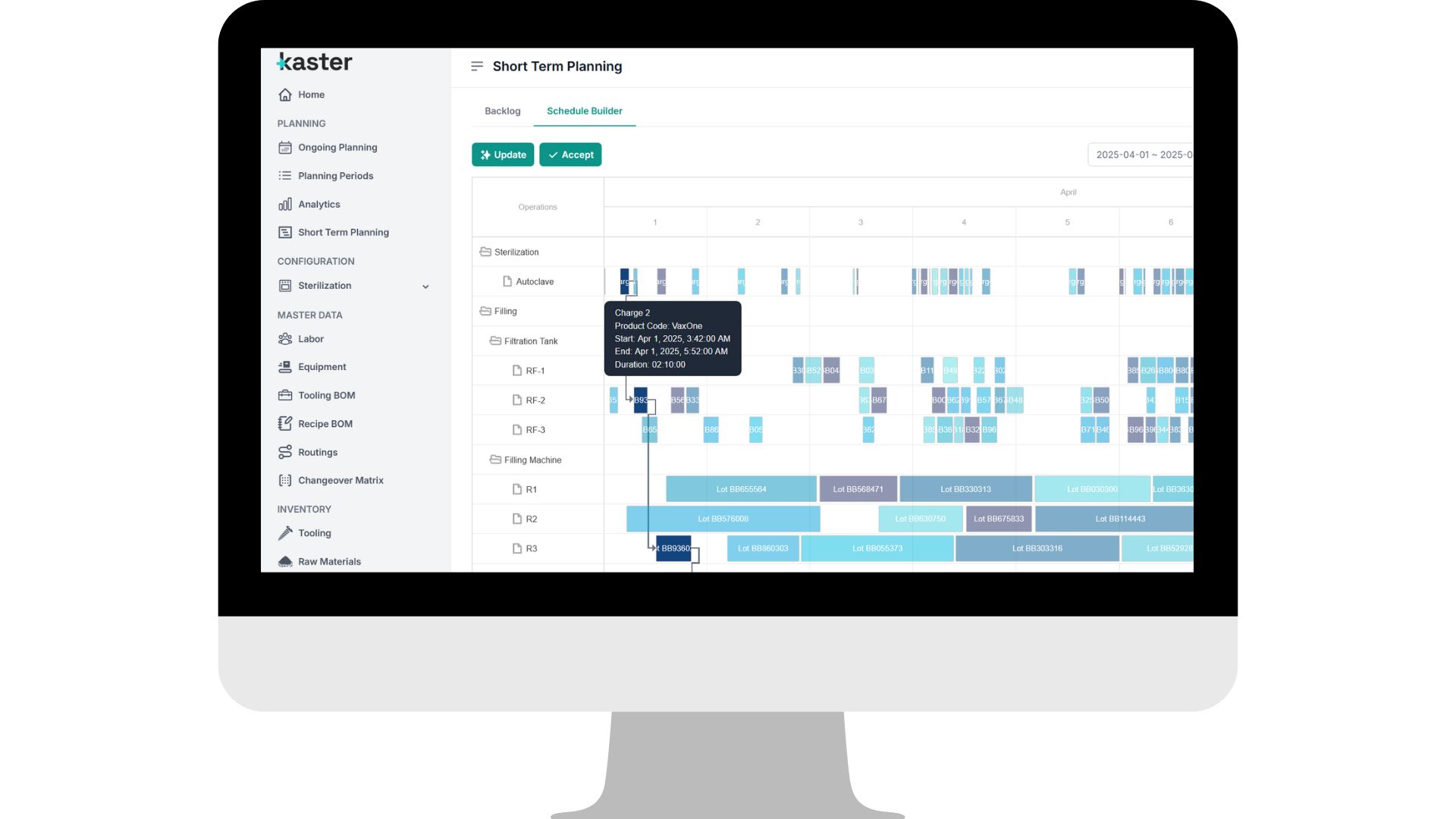 See it in action
See it in actionThe problems we solve
These aren’t software problems, they’re production problems. Schedules don’t hold, teams aren’t aligned, and capacity is lost in ways no ERP or Excel file can detect. We built AIM | Pace to solve the real issues behind firefighting, missed OTIF targets, and unstable execution, the ones every pharma plant deals with, but few talk about openly.
Why partner with us
We don’t just provide solutions to your toughest problems, we build long lasting innovation partnerships.

Co-develop with your teams.
We work side-by-side with your planners, operators, and quality teams to shape features that address real-world bottlenecks and fit seamlessly into your workflows.

Applied Research Pipeline
We continuously turns frontline manufacturing challenges, from changeovers to lab release sync, into deployable features that evolve with your operations.

Built-In GMP & Pharmaceutical Expertise
Purpose-built for sterile injectables, OSD, biologics, and more. Every feature is designed for large-scale workflows with compliance at the core.
Do any of these sound familiar?
Let’s walk through what’s happening on your floor, and how we’d fix it.

