AIM | Pace™ for Oral Solid Dosage
Plan high-volume, constraint-heavy production with confidence.
OSD lines are complex
Hundreds of SKUs, shared compression and coating equipment, changeover constraints and QA dependencies.
AIM | Pace brings order to that complexity, generating stable, right-the-first-time short-term plans that align people, equipment, and process, in real time.
 Book a demo
Book a demo"We can't keep a stable schedule across multiple SKUs and shifts."
Complex routings, thousands of constraints and misaligned teams keep your schedules in constant flux.
Shared assets (granulators, blenders, compression, coating) lead to bottlenecks
Cleaning and changeovers create unpredictable downtime
QA and lab dependencies make execution reactive
High SKU variety = complex routings and frequent re-priorization
Shift handoffs and misalignment create planning gaps
AIM | Pace gives you a real-time, constraint based planning engine made for OSD environments, keeping plans stable even when priorities shift.
 Book a demo
Book a demoAIM | Pace™
Coordinating changeovers, release timings, shared resources, and shifting priorities challenges even the best-run plants.
AIM | Pace turns that complexity into GMP-compliant daily and weekly production plans that adapt in real time to shop-floor realities.
Built for sterile injectables, OSD, biologics, non-sterile liquids, suspensions, creams, and more, it delivers stable operations, higher throughput, and reduced overtime, all without added CapEx.
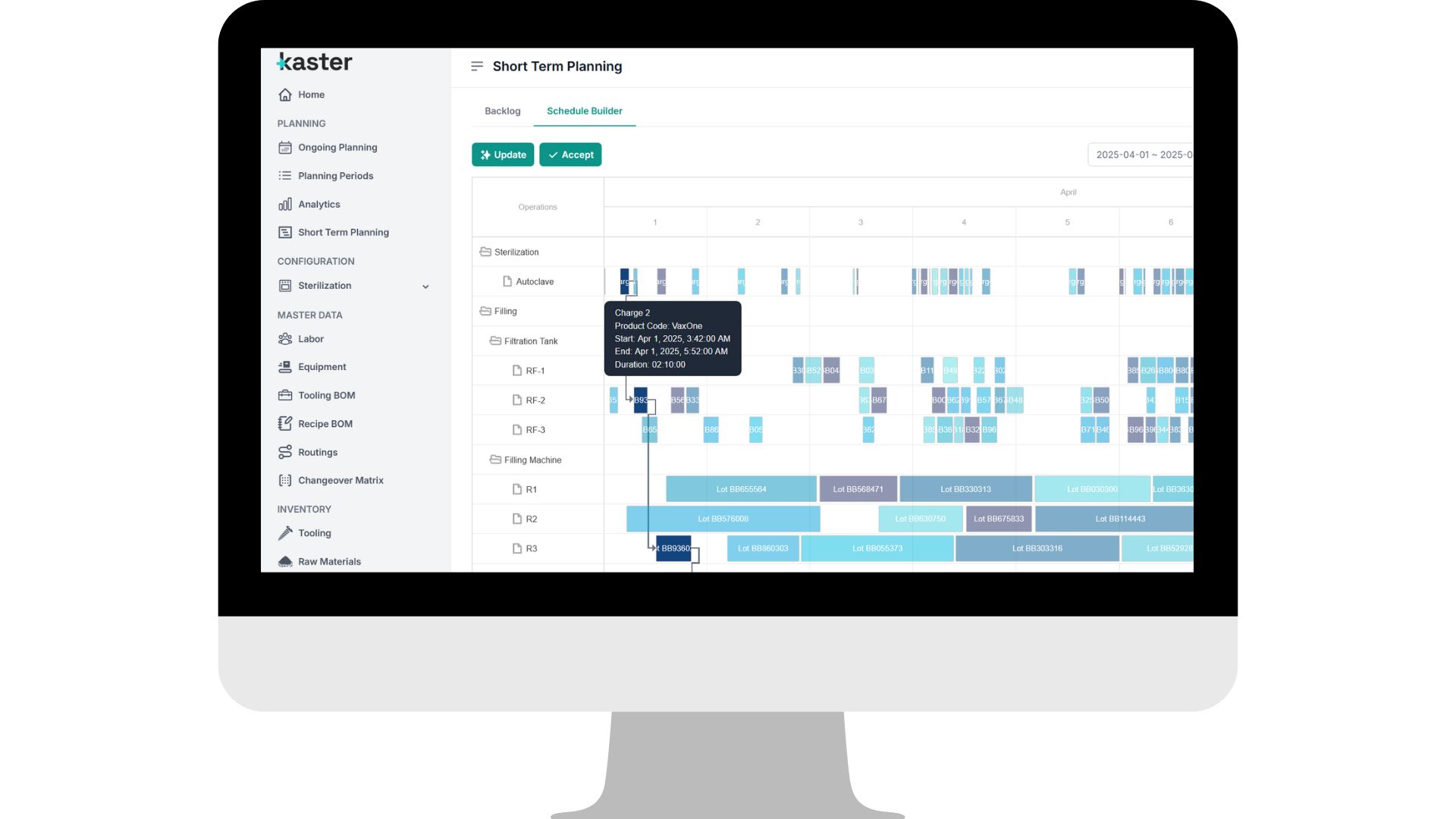 See it in action
See it in actionWhat we optimize in large-scale OSD environments
OSD may not be sterile, but it’s still heavily regulated. Cleaning validation, cross-contamination risks, operator certifications, and environmental zoning still matter. AIM | Pace integrates these constraints natively into the plan, so you don’t need workarounds or manual enforcement.

How OSD sites reclaim time, capacity and control
Kaster Technology's proprietary prescriptive AI has delivered quantifiable improvements across diverse pharma plants, proving that smarter scheduling drives both operational stability and business performance.

+10% Capacity Gain, unlocked without CapEx.

+20% more efficient workflows, validated at Canada’s largest sterile injectable site.

GMP-Compliant, Audit-Ready Execution, confidence in every plan.
Built for high-volume OSD manufacturing

Handles 100–500+ SKUs across multiple formats and product families

Multi-format and clients CDMOs or integrated pharma sites

Supports batching logic (campaigns, lot sizing, shift constraints)

Sustainability & ESG contribution

Equipment sitting idle, unnecessary cleaning, or night shift overruns don’t just cost time, they consume energy, ingredients, and human resources. AIM | Pace helps reduce this waste by improving plan accuracy, minimizing avoidable cleaning, and reducing equipment overuse.

Why partner with us
We don’t just provide solutions to your toughest problems, we build long lasting innovation partnerships.

Co-develop with your teams.
We work side-by-side with your planners, operators, and quality teams to shape features that address real-world bottlenecks and fit seamlessly into your workflows.

Applied Research Pipeline
We continuously turns frontline manufacturing challenges, from changeovers to lab release sync, into deployable features that evolve with your operations.

Built-In GMP & Pharmaceutical Expertise
Purpose-built for sterile injectables, OSD, biologics, and more. Every feature is designed for large-scale workflows with compliance at the core.
Bring stability to your OSD operations.
Generate plans that hold. Reduce changeovers. Deliver on time.

