AIM | Pace™ for sterile injectables manufacturing
Plan aseptic production with precision, and execute without surprises.
Stabilize production for complex sterile injectables manufacturing.
Sterile injectables production is time-sensitive, compliance-heavy, and operationally unforgiving.
AIM | Pace brings real-time coordination to every cleanroom and resource involved, ensuring your schedule holds under pressure.
 Book a demo
Book a demo"To produce sterile drugs, everything has to be right, or the batch is at risk.."
Complex routings, thousands of constraints and misaligned teams keep your schedules in constant flux.
Shared cleanroom assets:
Grade A/B rooms and support areas are often shared across lines, and one conflict can block the entire day.
Cleaning and changeovers:
Between campaign changes, cross-contamination risk, and validated cleaning procedures, cleaning windows block suite availability longer than expected.
Reactive Planning Under Pressure:
A missed sterilization cycle, late gowning, or QA hold can disrupt the entire campaign, with no easy way to adjust quickly.
QA and QC Lab Coordination:
Sampling, environmental monitoring, and batch record review often lag behind production, delaying downstream steps or release.
Shift Overlap and Role-Specific Tasks
Aseptic operators, setup techs, and QA reviewers have non-interchangeable roles, and aren’t always available when needed.
AIM | Pace™ builds GMP-compliant, conflict-free plans that hold, even in high-pressure aseptic environments..
 Book a demo
Book a demoAIM | Pace™
Coordinating changeovers, release timings, shared resources, and shifting priorities challenges even the best-run plants.
AIM | Pace™ turns that complexity into GMP-compliant daily and weekly production plans that adapt in real time to shop-floor realities.
Built for sterile injectables, OSD, biologics, non-sterile liquids, suspensions, creams, and more, it delivers stable operations, higher throughput, and reduced overtime, all without added CapEx.
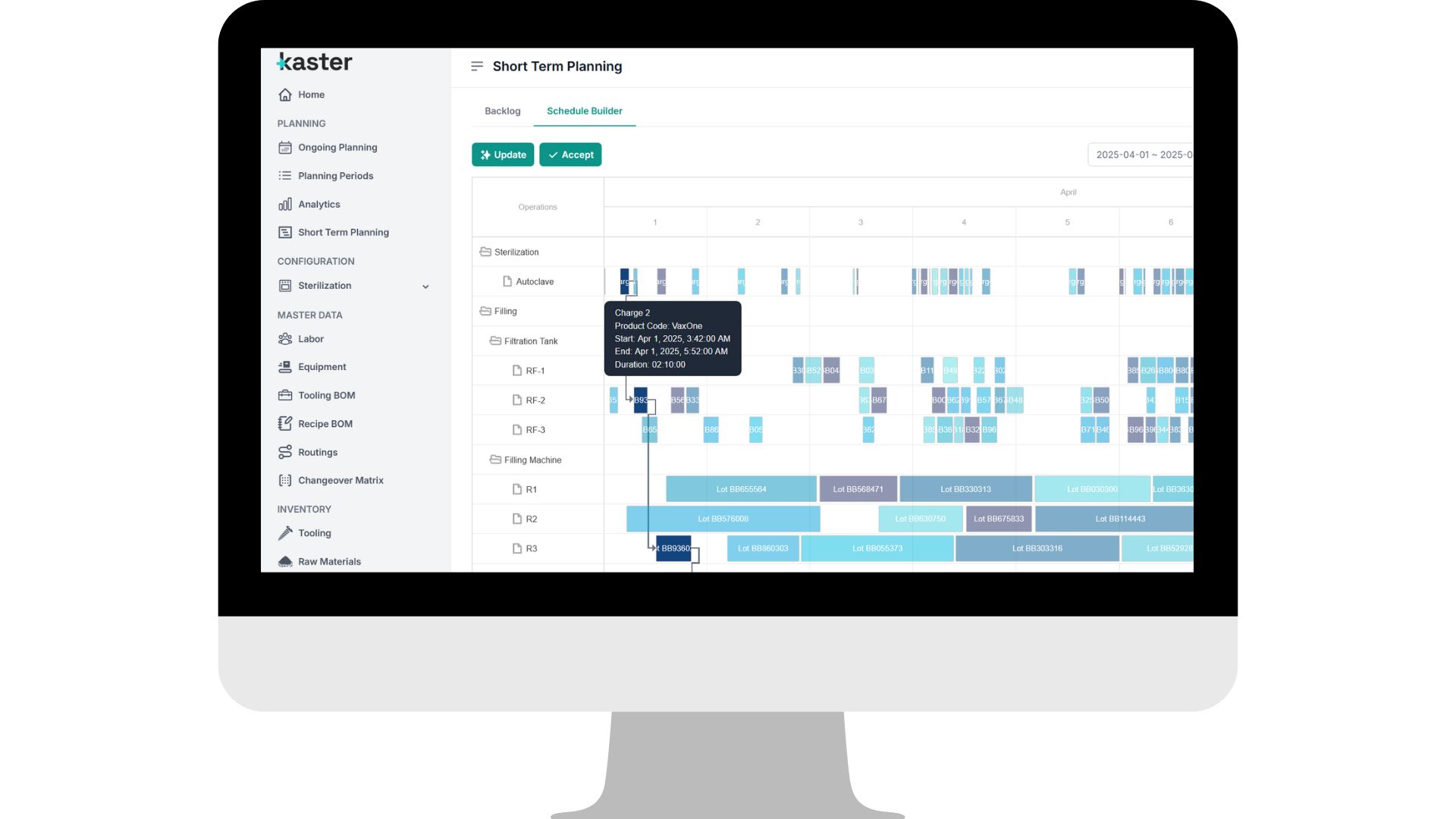 See it in action
See it in actionWhat we optimize in large-scale sterile injectables production environments
Sterile injectables production isn’t just complex, it’s deeply regulated. Cleaning validation protocols, cross-contamination controls, operator qualifications, and cleanroom zoning must all be enforced without fail. AIM | Pace™ embeds these GMP constraints directly into the planning logic, eliminating manual workarounds and reducing the risk of compliance gaps.

How sterile injectables sites reclaim time, capacity and control.
Kaster Technology's proprietary prescriptive AI has delivered quantifiable improvements across diverse pharma plants, proving that smarter scheduling drives both operational stability and business performance.

+10% Capacity Gain, unlocked without CapEx.

+20% more efficient workflows, validated at Canada’s largest sterile injectable site.

GMP-Compliant, Audit-Ready Execution, confidence in every plan.
Built for high-volume sterile injectables manufacturing

Handles 100–500+ SKUs across multiple formats and product families

Multi-format and clients CDMOs or integrated pharma sites

Coordinates filling/isolator/autoclave use, sterilization windows, and pre/post-op buffers

Sustainability & ESG contribution

Equipment sitting idle, unnecessary cleaning, or night shift overruns don’t just cost time, they consume energy, ingredients, and human resources. AIM | Pace helps reduce this waste by improving plan accuracy, minimizing avoidable cleaning, and reducing equipment overuse.

Why partner with us
We don’t just provide solutions to your toughest problems, we build long lasting innovation partnerships.

Co-develop with your teams.
We work side-by-side with your planners, operators, and quality teams to shape features that address real-world bottlenecks and fit seamlessly into your workflows.

Applied Research Pipeline
We continuously turns frontline manufacturing challenges, from changeovers to lab release sync, into deployable features that evolve with your operations.

Built-In GMP & Pharmaceutical Expertise
Purpose-built for sterile injectables, OSD, biologics, and more. Every feature is designed for large-scale workflows with compliance at the core.
Bring stability to your aseptic operations.
Generate plans that hold. Reduce changeovers. Deliver on time.

