We help pharmaceutical manufacturers align people, priorities, and production, in real time.
The daily chaos you know too well.
Complex routings, thousands of constraints and misaligned teams keep your schedules in constant flux.
OTIF delays and conflicts from
shared resources and changeovers
Lab and QC releases out of sync with production schedules
GMP limits and disconnected Excel/ERP slowing execution plant-wide
Large-scale, complex routings and timings hard to coordinate across departments
Legacy tools ignore these problems.
We solve them in seconds.

Where AI turns scheduling into measurable gains.
We built the prescriptive AI for pharmaceutical production, uniting teams, priorities, and equipment to master high-volume, complex scheduling decisions in real time.

Regulatory-Aware Scheduling
Automatically respects GMP rules, equipment constraints, and timing requirements to deliver the most efficient, compliant production sequence, every time.

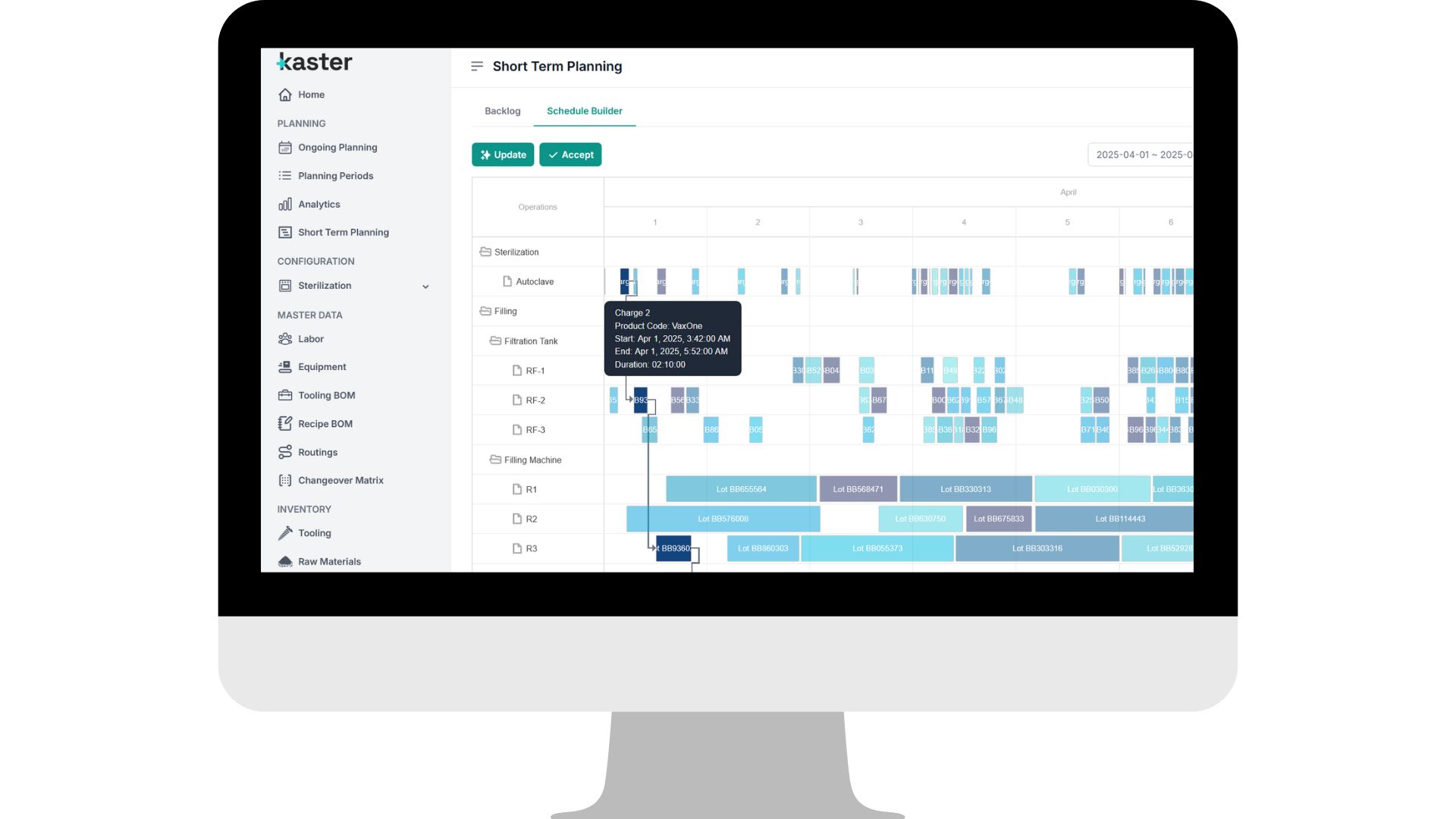
Detailed, Granular-Level Planning
Continuously adjusts production plans down to the minute, optimizing changeovers, routings, and equipment use to match real demand and priorities in every department.

Shared-Resource Orchestration
Coordinate rooms, lines, utilities, and people across departments to prevent conflicts and OTIF delays.

Lab & QA Release Synchronization
Align lab testing, QA release timings, and production schedules so batches move without unnecessary waiting.

Energy Usage Optimization
Optimize equipment use to cut energy consumption, lower operating costs, and reduce emissions, supporting your ESG goals.

Changeover & Campaign Optimization
Plan complex routings and sequence changeovers to reduce downtime and maximize throughput across dosage forms.
AIM | Pace™
Validated at one of Canada’s largest sterile injectable sites, designed to deliver more than 10% extra production capacity without new equipment.
What AIM | Pace™ Delivers
Executable GMP Daily Plans
Models every constraint: changeovers, campaign sequencing, line availability, labor, utilities, and shared equipment to deliver production-ready schedules.
Closed-Loop Scheduling
Adjusts automatically to real-time events such as delays, holds, or lab release timings to keep operations synchronized and stable.
Low-Friction Integration
Drives higher throughput and more reliable schedules without replacing or disrupting your existing ERP or MES.
Together, these capabilities let drug manufacturers run stable, high-throughput schedules under GMP constraints, unlocking hidden capacity and reducing delays without adding shifts or new equipment.

How drug makers reclaim time, capacity and control
Kaster Technology's proprietary prescriptive AI has delivered quantifiable improvements across diverse pharma plants, proving that smarter scheduling drives both operational stability and business performance.

+10% Capacity Gain, unlocked without CapEx.

+20% more efficient workflows, validated at Canada’s largest sterile injectable site.

GMP-Compliant, Audit-Ready Execution, confidence in every plan.
Why partner with us
We don’t just provide solutions to your toughest problems, we build long lasting innovation partnerships.

Co-develop with your teams.
We work side-by-side with your planners, operators, and quality teams to shape features that address real-world bottlenecks and fit seamlessly into your workflows.

Applied Research Pipeline
We continuously turns frontline manufacturing challenges, from changeovers to lab release sync, into deployable features that evolve with your operations.

Built-In GMP & Pharmaceutical Expertise
Purpose-built for sterile injectables, OSD, biologics, and more. Every feature is designed for large-scale workflows with compliance at the core.


